News & Events
Department of Pharmacology and Toxicology
Evidence that engineered muscle could patch up failing hearts
Heart failure, affecting 64 million people globally, remains intractable due to the heart’s inability to regenerate after injury. Current therapies cannot repair damaged myocardium, leaving transplantation as the sole option for severe cases. This study, led by Prof. Dr. Wolfram-Hubertus Zimmermann and recently published in Nature, introduces engineered heart muscle (EHM) allografts—lab-grown patches of induced pluripotent stem cell (iPSC)-derived cardiomyocytes—as a breakthrough approach for myocardial remuscularization. Preclinical trials in rhesus macaques demonstrated that EHM allografts, implanted under immune suppression, sustainably integrated into the heart, thickened ventricular walls by up to 15%, and improved pumping efficiency without arrhythmias or tumors. These findings enabled regulatory approval for the first-in-human BioVAT-HF trial, where a 46-year-old patient with advanced heart failure received ten EHM patches. Post-transplant analysis confirmed graft retention, vascularization, and maturation.
The EHM’s design—electrically synchronized and metabolically efficient cells embedded in collagen—mimics juvenile heart tissue, enabling mechanical integration with host myocardium. This “mechano-electrical coupling” avoids arrhythmogenic risks seen in injected cardiomyocytes.
While the exact mechanisms remain unclear, the grafts’ surface placement and mechanical conditioning by the recipient’s heartbeat likely drive functional synchronization. This proof-of-concept bridges regenerative medicine and clinical care. The study establishes a translational blueprint for iPSC-based therapies, offering hope for scalable myocardial repair beyond palliative care or scarce donor organs.
Breakthrough stem-cell patches strengthened a woman’s failing heart
A groundbreaking clinical trial led by Prof. Dr. Wolfram-Hubertus Zimmermann demonstrated the potential of stem cell-derived engineered heart muscle (EHM) patches to stabilize advanced heart failure. In a first-in-human case, a 46-year-old woman with post-heart attack heart failure received ten lab-grown patches (400 million cells total) implanted onto her heart’s surface in 2021. The procedure, combined with immunosuppressive therapy, stabilized her condition for three months until a heart transplant became available. Post-transplant analysis revealed the patches had integrated with host tissue, forming functional blood vessels without arrhythmias or tumors.
Parallel preclinical studies in rhesus macaques showed that larger patches (up to 200 million cells) thickened heart walls by 15% and improved pumping efficiency by 10% over six months, validating safety and efficacy.
The patches, created from induced pluripotent stem cells (iPSCs) differentiated into cardiomyocytes and embedded in collagen, were delivered via minimally invasive surgery. This approach aims to bridge the critical gap for patients awaiting transplants, addressing a global shortage of donor hearts.
While the treatment is not a permanent replacement for transplants, it offers a viable interim solution for severe heart failure patients under palliative care. Ongoing trials with 15 participants aim to refine patch design and reduce immunosuppression needs, marking a pivotal step toward scalable cardiac remuscularization therapies.
Link to full article in Nature
Mechanical heart support promotes regeneration of heart muscle cells
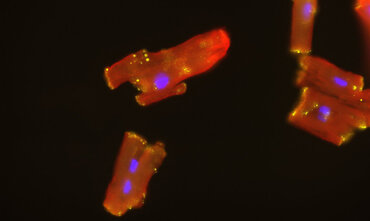
A study team led by Olaf Bergmann from the Institute of Pharmacology and Toxicology at the University Medical Center Göttingen (UMG) has found that patients with advanced heart failure experience significantly increased regeneration of their heart muscle cells through the use of a left ventricular assist device (LVAD), a mechanical pump for cardiac support. The results were published in the scientific journal ‘Circulation’.
The study analysed tissue samples of hearts removed from 52 patients with advanced heart failure. 28 of these patients were treated with a left ventricular assist device (LVAD) before heart removal, i.e. the weakened heart was mechanically supported before removal. The other 24 patients were not treated prior to heart removal. A new heart was transplanted into all 52 patients after the heart was removed.
To measure the renewal of the heart muscle cells, the researchers used an innovative method: they analysed the amount of radioactive carbon, the isotope C-14, in the DNA of the heart muscle cells. This radioactive carbon was released into the atmosphere during nuclear weapons tests in the 1950s and 1960s. Traces of this carbon can still be detected in the DNA of humans today, and therefore also in the DNA of heart muscle cells. Since the ban on nuclear weapons tests, the quantities of atmospheric radiocarbon in DNA have decreased again. Based on the amount of this radioactive carbon detected in the DNA of each patient, Dr Bergmann and his team of researchers were able to use a mathematical model to calculate the age of the heart muscle cells and thus the rate of renewal.
In the heart tissue samples from 15 of the 28 patients with an LVAD, a six times higher rate of heart muscle cell renewal was measured compared to heart tissue samples from healthy individuals. The other 13 patients with LVAD did not respond to treatment and, like the 24 untreated patients with severe heart failure, showed 18 to 50 times lower regeneration compared to healthy hearts. The reason for the improvement with an LVAD is probably the mechanical relief, which reduces the pressure on the weakened heart and enables better recovery of heart function. The result is also due to so-called ‘reverse remodelling’, in which the pathological enlargement of the heart muscle is partially reversed. ‘The LVAD allows the heart to recover. This in turn improves energy efficiency and thus presumably promotes the regeneration of the heart muscle cells, which could not be observed in the samples without mechanical support,’ says Dr Bergmann.
‘The determination of radioactive carbon enabled us to precisely determine the renewal of heart muscle cells. The results indicate that, despite the low renewal rates in severe heart failure, there is considerable regenerative potential that could be utilised therapeutically. These findings open up new perspectives for the treatment of heart disease and could in future lead to more effective therapies that promote heart regeneration,’ adds Prof. Dr Wolfram-Hubertus Zimmermann, Director of the Institute of Pharmacology and Toxicology at UMG.
Link to the UMG Press release (in German)
Original Publication:
Wouter Derks, Julian Rode, Sofia Collin, Fabian Rost, Paula Heinke, Anjana Hariharan, Lauren Pickel, Irina Simonova, Enikő Lázár, Evan Graham, Ramadan Jashari, Michaela Andrä, Anders Jeppsson, Mehran Salehpour, Kanar Alkass, Henrik Druid, Christos P. Kyriakopoulos, Iosif Taleb, Thirupura S. Shankar, Craig H. Selzman, Hesham Sadek, Stefan Jovinge, Lutz Brusch, Jonas Frisén, Stavros Drakos, Olaf Bergmann: A latent cardiomyocyte regeneration potential in human heart disease. Circulation. November 21st, 2024. DOI: 10.1161/CIRCULATIONAHA.123.067156
Preis der Deutschen Hochschulmedizin 2024

This year`s German University Medicine Award (Preis der Deutschen Hochschulmedizin) goes to a research team from the University Medical Centre Göttingen (UMG) and the University Medical Centre Schleswig-Holstein (UKSH). The team has developed a globally unique approach with the ‘heart patch’ - a therapy using stem cells to repair the heart muscle and strengthen the heart. The patch was tested on human volunteers for the first time in the BioVAT-DZHK20 clinical trial funded by the DZHK and Repairon GmbH. The ‘heart patch’ is a prime example of translational research that brings results from the laboratory into clinical application.
The approach is unique in the world. The ‘heart patch’ made from heart muscle cells is applied directly to the heart in a gentle and minimally invasive way. The researchers use induced pluripotent stem cells (iPSC) to develop functional heart muscle and connective tissue cells. The world's first transfer of an iPSC-based heart repair approach via rhesus monkeys to humans is a special team effort by basic scientists, translational researchers and clinicians from the UMG and the UKSH.
In a unique approach, the researchers were able to show that a heart which has undergone damage due to a heart attack, can be repaired by implanting a heart patch. In addition, an improvement in heart function can be achieved in this way, and there are no adverse side effects. The world's first clinical trial of permanent heart muscle reconstruction using human stem cells was approved by the Paul Ehrlich Institute in December 2020. Since March 2021, patients have been treated as part of the BioVAT-HF-DZHK20 (Biological Ventricular Assist Tissue in Terminal Heart Failure) clinical trial.
The jury recognised the interdisciplinary teamwork and innovative strength of the project. The German University Medicine Prize is awarded annually by the Medical Faculty Association (MFT) and the Association of University Hospitals in Germany (VUD). In addition to team performance in university medicine, it recognises in particular the innovation and translation of research projects for patient care as well as the social impact of medical achievements.
The award ceremony will take place during the Day of University Medicine on 28 November 2024 in Berlin. The prize is endowed with 25,000 euros.
Press release of the "Deutsche Hochschulmedizin" e.V.
Video on the Heart Patch (in German)
Habilitation Prize for Malte Tiburcy

Awardees of the "Habilitationspreis des Vereins der Freunde und Förderer der Medizinischen Fakultät der Georgia Augusta zu Göttingen e.V., WS 23/24" are PD Dr. Malte Tiburcy (Dep. of Pharmacology and Toxicology) and PD Dr. Moritz Maximilian Hollstein (Dep. of Dermatology, Venereology and Allergology). PD Dr. Malte Tiburcy recieves the prize for his habilitation thesis: "Humane Muskelgewebe für die translationale Pharmakologie".
Webpage Verein der Freunde und Förderer der Medizinischen Fakultät der Georgia Augusta zu Göttingen e.V.
Wolfram-Hubertus Zimmermann Distinguished Lecturer at Eurolife Network

The "Eurolife Network of European Universitits in Life Sciences" celebrated its 25th anniversary with a summit at the University Medical Center Göttingen. Prof. Dr. Wolfram-Hubertus Zimmermann was distinguished speaker at this event. The event featured the Eurolife Semi-Annual Academic Symposium on “Advanced Therapies for Cardiovascular Disease,” where distinguished experts from partner institutions delivered groundbreaking insights and were honored with the prestigious Eurolife Medals for their contributions to advancing cardiovascular research.
Ausstellung "Herz & Hirn - gemeinsam verstehen"

Herz & Hirn — gemeinsam verstehen
18. April – 20. Oktober 2024 | Forum Wissen
Berliner Straße 28, 37073 Göttingen
Öffnungszeiten: Di — So von 10 — 18 Uhr
Eintritt frei!
Wie funktioniert ein „Pflaster“ für das Herz? Wie kann man mit Hilfe von Licht hören? Und wie verläuft der Weg von der Grundlagenforschung zur Diagnose und zur Therapie? Diesen Fragen geht die Ausstellung „Herz & Hirn – gemeinsam verstehen“ auf den Grund.
Die Ausstellung präsentiert modernste Mikroskopietechniken, mit denen Forschende in Zellen und Gewebe „hineinschauen“ können. Besucher*innen sind eingeladen zu einer Reise durch die Bilderwelten moderner Mikroskopie. Eigens für die Ausstellung produzierte Filme, interaktive Anwendungen und Tastmodelle bieten auf ca. 360 qm vielfältige Einblicke in die interdisziplinäre Forschung der Wissenschaftler*innen des Exzellenzclusters „Multiscale Bioimaging: von molekularen Maschinen zu Netzwerken erregbarer Zellen (MBExC)“ an der Georg-August-Universität und der Universitätsmedizin Göttingen. Das Exzellenzcluster untersucht die Zusammenhänge von Herz- und Gehirnerkrankungen mit neuesten bildgebenden Verfahren.
Überraschende Gemeinsamkeiten entdecken!
Im Fokus des MBExC stehen die elektrisch erregbaren Herzmuskel- und Nervenzellen, die überraschend viele Gemeinsamkeiten aufweisen. Ziel ist es, diese zu erforschen, um beide Systeme und ihre Funktionen ganzheitlich vom Molekül bis zum Organ zu untersuchen und zu verstehen. Kleinste Zellbausteine und Netzwerke ermöglichen die komplexen Funktionen eines Organs und spielen bei der Entstehung von Erkrankungen eine wesentliche Rolle. Auf dieser Grundlage werden neue Ansätze für die Diagnose und Therapie von Erkrankungen des Herzens, des Gehirns oder beider Organe entwickelt.
Die Ausstellung nimmt die Besucher*innen mit auf eine Reise durch die Entstehungs- und Entwicklungsprozesse von wissenschaftlichen Erkenntnissen und verdeutlicht die gesellschaftliche Relevanz dieser Forschung, die den Menschen in den Mittelpunkt stellt.
8th Annual NIH PCTC Cardiovascular Bioengineering Symposium

"The George and Angelina Kostas Research Center for Cardiovascular Nanomedicine Symposium" and the 8th Annual NIH PCTC "Cardiovascular Bioengineering Symposium" will take place May 22-25 in Houston, Texas: https://cvbe2024tmc.org/
Wolfram Zimmermann will speak on "Heart Repair with Tissue Engineered Biological Ventricular Assist Tissue".
Find other interesting speakers on: https://cvbe2024tmc.org/agenda/
Date: May 22-25, 2024
Place: Hybrid: Houston Methodist Research Institute, Texas Medical Center, Houston, Texas / virtual participation:
The 8th National Institutes of Health Progenitor Cell and Translational Medicine in Cardiovascular Bioengineering 2024 (PCTC CVBE 2024) Symposium is a hybrid event offering an in-person and virtual component for participants who are unable to travel to the U.S.
Registration: https://learn.houstonmethodist.org/content/george-and-angelina-kostas-research-center-cardiovascular-nanomedicine-8th-annual
Vortrag: Herztöne | Herzpflaster: Lässt sich das Herz reparieren?
Am 7. Mai 2024 spricht Prof. Dr. Wolfram-Hubertus Zimmermann zum Thema "Herzpflaster".
Thema: Herzpflaster: Lässt sich das Herz reparieren?
Referent: Prof. Dr. Wolfram-Hubertus Zimmermann, Direktor des Instituts für Pharmakologie und Toxikologie
Datum: Dienstag, 7. Mai 2024
Zeit: ab 18 Uhr
Ort: StartRaum, Friedrichstraße 3/4, 37073 Göttingen
Anmeldung: Öffentlichkeitsarbeit des Herzzentrums |
Weitere Informationen unter: herzzentrum.umg.eu/herztoene
Podiumsdiskussion "Herzpflaster"


Podiumsdiskussion und Pressekonferenz "Zwei Jahre mit Herzpflaster, Patient berichtet über Erfahrungen" (18. März 2024)
Die Studie BioVAT-HF-DZHK20 ist die weltweit einzige klinische Studie, in der Patient*innen mit Herzschwäche künstliches Herzgewebe implantiert wird, um den Herzmuskel dauerhaft zu stärken. Die Studie ist ein Exempel für die translationale Forschung in und aus Göttingen: Vom Labor bis in die klinische Anwendung. Unser Ziel ist es, das Herzpflaster als Therapieverfahren für Patient*innen mit fortgeschrittener Herzschwäche zu etablieren – und das weltweit.
Bisher wurden 12 Patient*innen mit dem Herzpflaster behandelt, jeweils sechs an der Universitätsmedizin Göttingen (UMG) und dem Universitätsklinikum Schleswig-Holstein (UKSH).
Im Mittelpunkt der Podiumsdiskussion steht ein Patient, dem im April 2022 in Lübeck erstmalig die Höchstdosis an künstlichem Herzgewebe implantiert wurde. Nun berichtet er öffentlich von seinen Erfahrungen „2 Jahre mit Herzpflaster“.
Die Podiumsdiskussion wurde von Dr. Thomas Ramge, Sachbuchautor, Keynote-Speaker und Moderator, geleitet.
Teilnehmer:
- Prof. Dr. Stephan Ensminger, Direktor der Klinik für Herz- und thorakale Gefäßchirurgie, Universitätsklinikum Schleswig-Holstein (UKSH), Campus Lübeck
- Dr. Lothar Germeroth, Geschäftsführer der Repairon GmbH
- Prof. Dr. Ingo Kutschka, Direktor der Klinik für Herz-, Thorax- und Gefäßchirurgie, UMG
- Prof. Dr. Michael P. Schön, Ständiger Vertreter des Vorstands 1 der UMG
- Prof. Dr. Tim Seidler, Stv. Direktor der Abteilung Kardiologie, Kerckhoff Klinik GmbH
- Prof. Dr. Wolfram-Hubertus Zimmermann, Leiter des Instituts für Pharmakologie und Toxikologie, UMG
Pressemeldung der UMG zur Veranstaltung: https://www.umg.eu/news-detail/news-detail/detail/news/zwei-jahre-mit-herzpflaster-patient-berichtet-ueber-erfahrungen/
Video von SAT.1 Regional: https://www.sat1regional.de/herzpflaster-universitatsmedizin-gottingen-setzt-patienten-herzmuskelpraparat-aus-stammzellen-ein/
Bericht in der HNA: https://www.hna.de/lokales/goettingen/goettingen-ort28741/der-weltweit-erste-patient-mit-umg-herzpflaster-berichtet-92900712.html
Bericht im NDR: https://www.ndr.de/nachrichten/schleswig-holstein/Neue-Therapie-Mit-dem-Herzpflaster-gegen-Herzmuskelschwaeche-,herzmuskel136.html
Ringvorlesung 2023/24: „Herz und Hirn gemeinsam im Fokus“

„Herz und Hirn gemeinsam im Fokus“ (Heart and Brain Together in Focus) is the title of the public lecture series of the University of Göttingen and the Lower Saxony Academy of Sciences and Humanities in Göttingen in the winter semester 2023/2024. In this series, Göttingen Cluster of Excellence "Multiscale Bioimaging" (MBExC) scientists give comprehensive insights into the unique research approach of the Cluster.
On Tuesday, Dec. 05, 2023, Wolfram Zimmermann spoke on "Herzpflaster: Hilfe für ein schwaches Herz".
On Tuesday, Jan. 23, 2024, Niels Voigt gave a talk on "Herzbeben - Wenn das Herz außer Takt gerät...".
Programm of the "Ringvorlesung": https://s.gwdg.de/06jc6q
The talks will take place at 18:15 pm at the Aula, Wilhlemsplatz 1. The recordings of the lectures will be broadcast one week later on Wednesdays at 12 noon on StadtRadio Göttingen (107.1 MHz). In addition, they will be available in the long term as a video recording on the University’s YouTube channel as well as on the YouTube channel of the MBExC.
On the MBExC: https://mbexc.de/
Heart patch in clinical trial: dose finding completed
The world's only clinical trial in patients with heart failure to implant artificial heart tissue to permanently strengthen the heart muscle has reached the first important milestone: The determination of the maximum safe dose has been completed after treatment of 10 patients.
The clinical trial is evaluating the safety and efficacy of iPSC -derived engineered human myocardium (EHM) as Biological Ventricular Assist Tissue (BioVAT) in Advanced Heart Failure (NCT04396899). The determination of the maximum safe dose is now completed after treating 10 patients. A total of 35 patients will from now on be treated with artificial heart tissue from 800 million heart cells in order to gain insights into the efficacy of the heart patches. The study is preceded by 25 years of preclinical development. The completion of BioVAT-HF with 35 patients treated with the safe maximal dose is expected in 2025. The BioVAT-HF-DZHK20 study is being conducted at the University Medical Center Göttingen (UMG) and the University Hospital Schleswig-Holstein (UKSH), Lübeck Campus, and is funded by the German Center for Cardiovascular Research (DZHK) and Repairon GmbH.
To the UMG Press Release (in German)
To the DZHK Press Release (in German)
To the Repairon Press Release
Contact:
University Medical Center Göttingen, University of Göttingen
Dep. of Pharmacology and Toxicology
Prof. Dr. Wolfram-Hubertus Zimmermann
Robert-Koch-Straße 40, 37075 Göttingen
Telefon 0551 / 39-65781, Fax 0551 / 39-5699
Email:
Preclinical testing of gene therapies: researchers from Göttingen produce human skeletal muscles

Diseases of the skeletal muscles often lead to muscle weakness and, due to decreasing mobility, to severe limitations in the quality of life. Duchenne muscular dystrophy has a particularly severe course. The muscle weakness already appears in childhood and progresses rapidly. Patients become wheelchair dependent, have breathing problems and severely reduced life span. Current therapies can mitigate the progression, but do not provide a cure. Using cardiac and skeletal muscle models developed from stem cells at UMG's Institute of Pharmacology and Toxicology, researchers hope to develop precision therapeutics for the treatment of severe muscle diseases.
In a recently published paper, the authors report on how they produce skeletal muscle in the laboratory that can be used to simulate normal human muscle development, natural processes for muscle recovery (muscle regeneration), and disease processes of muscle disease.
VIDEO | Durchbruch in der Gentherapie: Forscher aus Göttingen stellen menschliche Skelettmuskeln her - SAT.1 REGIONAL (sat1regional.de) (21.02.2023,17:30, SAT.1 REGIONAL)
Link to the UMG Press release: https://www.umg.eu/news-detail/news-detail/detail/news/muskelerkrankungen-besser-erforschen-und-behandeln/
Original Publication: Shahriyari M, Islam MR, Sakib SM, Rinn M, Rika A, Krüger D, Kaurani L, Gisa V, Winterhoff M, Anandakumar H, Shomroni O, Schmidt M, Salinas G, Unger A, Linke WA, Zschüntzsch J, Schmidt J, Bassel-Duby R, Olson EN, Fischer A, Zimmermann WH, Tiburcy M. Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy. J Cachexia Sarcopenia Muscle. 2022 Oct 18. doi: 10.1002/jcsm.13094. Epub ahead of print. PMID: 36254806.
Contact:
Dr. Malte Tiburcy
Department of Pharmacology and Toxicology
Tel. 0551/ 39-20729
Robert-Koch-Straße 40, 37075 Göttingen
m.tiburcy(at)med.uni-goettingen.de
Research without animal testing: BMEL-Tierschutzforschungspreis (Animal Welfare Research Award) goes to UMG and IMES

Researchers from The University Medical Center Göttingen and Leibniz University Hannover were successful in the 41st call for entries for the Animal Welfare Research Award from the German Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft, BMEL).
The winners of the Animal Welfare Research Award 2022 are Dr. Tim Meyer from the Department of Pharmacology and Toxicology at the University Medical Center Göttingen and M.Sc. Leon Budde from the Institute of Mechatronic Systems (IMES) at Leibniz University Hannover.
By optimizing and automating Engineered Heart Muscles (EHM) developed under the leadership of Prof. Zimmermann, they are making this novel technology attractive for large scale academic and commercial drug development. EHM-based high-throughput methods help to identify new drugs with better efficacy and fewer side effects already at laboratory level, so they can be quickly brought into clinical application. The goal of performing preclinical development on human stem cell-derived EHM is to eliminate animal testing as much as possible.
The prize is endowed with 25,000 € and will be used to subject the automated tissue production in the patented multititer format to a "stress test" with reference substances.
The prize is being awarded by the German Federal Ministry of Health since 1980 and by the German Federal Ministry of Food and Agriculture (BMEL) since 2001 for the development of scientific alternative methods to animal experiments.
Dr. Tim Meyer, group leader at the Institute of Pharmacology and Toxicology at the University Medical Center Göttingen, conducts research on Decentralized Laboratory Automation & Extrusion 3D Bioprinting of Human Heart Muscle.
Link to the Press release of the BMEL (in German): https://www.bmel.de/SharedDocs/Pressemitteilungen/DE/2022/164-tierschutzforschungspreis.html
Link to the Press release of the UMG (in German): https://www.umg.eu/presse/news-detail/news-detail/detail/news/tierschutzforschungspreis-2022-fuer-junge-wissenschaftler-aus-goettingen-und-hannover/
Contact:
Dr. Tim Meyer, Department of Pharmacology and Toxicology, University Medical Center Göttingen
phone: +49 551 395879 mail: tim.meyer(at)med.uni-goettingen.de
NIH DZHK Cardiovascular Bioengineering (CVBE) Symposium 2022

June 9th-June 11th
German Primate Centre, Kellnerweg 4, 37077 Göttingen
Alte Mensa, Wilhelmsplatz 3, 37073 Göttingen
Aula, Wilhelmsplatz 1, 37073 Göttingen
Cardiovascular bioengineering is developing rapidly, aiming at offering solutions for heart failure repair. A fundamental understanding of disease mechanisms as well as the realization that heart failure is a multifaceted disease entity is key for the translation of innovative, individualized heart failure therapies.
Depending on underlying disease mechanisms and disease states, classical pharmacological approaches, biologicals, gene and cell therapy approaches or combined therapies may have to be considered for optimal outcomes. Safe and effective delivery of innovative therapeutics is a key challenge addressed by innovative bioengineering approaches. Early clinical studies are emerging to test preclinical innovations.
We are proud to have attracted the attention of world leaders in fundamental and translational heart failure research and at the same time provide a platform for Young Investigators to present their innovative research. We expect vibrant discussions as to the identification of novel therapeutic targets and strategies to advance the state-of-the-art experimental knowledge into clinical applications.
Organizers: Jay Zhang, Wolfram Zimmermann
- Link to the Program PDF
- Link to the DZHK Page