Facilities
Our Methods and Technologies
Engineering of Tissue Organoids
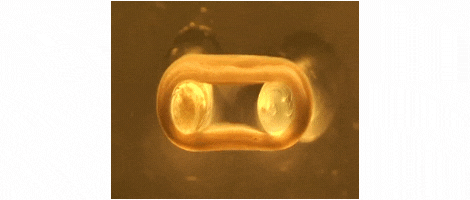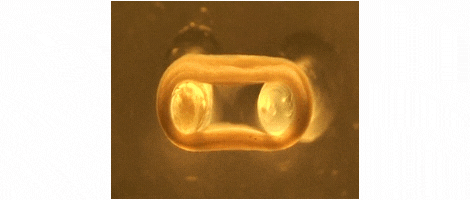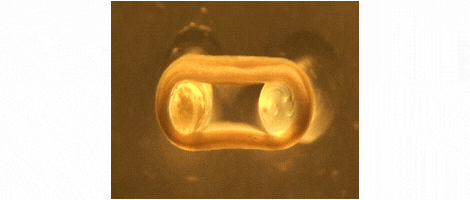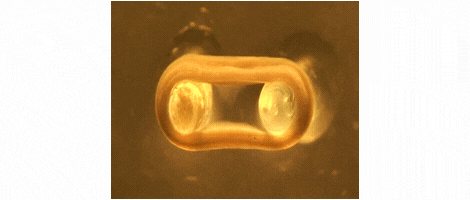
We are developing technologies for tissue engineering with a focus on engineered heart and skeletal muscle for drug testing, disease simulation, and organ repair. We focus on rat, mouse, and human models.

Stem Cell Culture & Differentiation
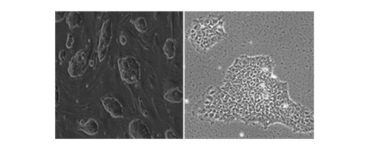
We perform culture and cardiac differentiation of mouse, rhesus monkey, and human embryonic stem cell (ESC), induced pluripotent stem cell (iPSC), and parthenogenetic stem cell lines (PSC). Human iPSC are generated via the Stem Cell Unit (SCU).
Stem Cell Unit
The Stem Cell Unit is a non-commercial service platform at the "Universitätsmedizin Göttingen" (UMG). It is funded by the "Deutsches Zentrum für Herz- Kreislauf-Forschung" (DZHK) and the UMG.
It aims to provide patient-specific induced pluripotent stem (iPS) cells to the research community at the Göttingen Research Council (GRC) and its partners. All iPS cell generations follow established and validated SOPs.
Contact

contact information
- telephone: +49 551 3964280
- e-mail address: lukas.cyganek(at)gwdg.de
Muscle Pharmacology and Biophysics
We employ isometric force measurements and dynamic mechanical analyses to define tissue properties of engineered and natural muscle tissue.




3-Dimensional Prototyping
We are using CAD design and 3D printing to develop prototypes, mold casts, and support materials for tissue engineering, cell culture, electrophysiology and other applications.


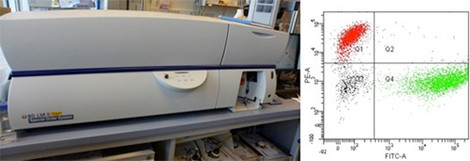
Flow Cytometry

Our lab provides access to a Becton Dickinson LSRII cytometer which contains 5 lasers (355 nm, 405 nm, 488 nm, 561 nm, and 640 nm excitation) and up to 18 detectors for multicolor experiments.
Macro- and Microscopy
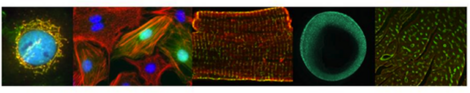


Next to macroscopic methods, we use standard light, confocal, and 2-photon microscopy in fixed and life samples under controlled environmental conditions; equipment: IX-81 with life cell chamber (Olympus), SteREO Lumar.V12 (Zeiss), LSM 710 NLO (Zeiss), Dual-Ultima L System (SciMedia), TCS LSI (Leica), Functional Optical Imaging (Ionoptix, Andor), Spectral Karyotyping (SKY).
High Content Screens



High-throughput recordings of cellular electrophysiology
Our new technology platform provides access to a patch-clamp robot “SyncroPatch 384” from Nanion Technologies. This enables high-throughput recordings of cellular electrophysiology of isolated cardiomyocytes and neurons.
We utilize several higher content screening platforms to analyze absorbance, fluorescence intensity, fluorescence polarization, time-resolved fluorescence, and luminescence; equipment: FlexStation 3 (Molecular Devices), GloMax® 96 Microplate Luminometer (Promega), Cellavista (SynenTec GmbH).



Histology
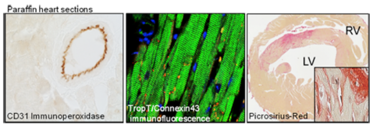
We perform standard histological analyses, immunohistochemistry, and immunofluorescence labeling in paraffin (1-4 µm, Leica RM 2255), cryo (6-20 µm, Leica CM 3050S), and vibratome (20-150 µm, Leica VT 1000 S) sections.
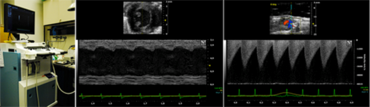
Small Animal Imaging and Interventions
We perform comprehensive cardiovascular phenotyping in rodent models with myocardial infarction and heart failure by ultrasound biomicroscopy (Vevo 2100 Imaging Systems, VisualSonics) and minimal invasive telemetry (DSI).