About us
The main objective of our research activities is to explore fundamental cardiovascular biology. Our team is specifically interested on the function of the transcriptional and post-transcriptional machinery of the developing, diseased and normal myocardium. A special focus is placed on evolutionary conserved endogenous mechanisms including the nuclear Wnt signaling complexes and their role in tissue remodeling. Our models comprise preclinical animal and humanized models. We aim to exploit our gained knowledge and expertise to define disease-specific and personalized therapeutic options and to ultimately establish CRISPR-based endogenous transcription engineering to therapeutically program a homoeostatic heart. This is feasible by embedding the process in a leading, cutting-edge cardiovascular research environment using state-of-the-art technology.

contact information
- telephone: +49 551 3968186
- e-mail address: laura.zelarayan(at)med.uni-goettingen.de
Address
Robert-Koch-Straße 40
37075 Göttingen
ORCID: 0000-0002-9001-0346
The cardiac transcriptional Wnt-complex
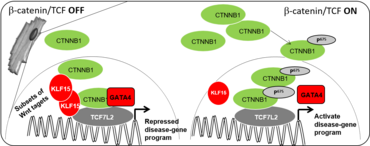
Transgenic activation of Wnt/β-catenin signaling results in cardiac dysfunction. Accordingly, preserved function after ischemic and pressure overload was observed upon inhibition of Wnt/b-catenin signaling in the adult mouse heart. Our team has demonstrated that aberrantly high Wnt/β−catenin/TCF-mediated activity in adult cardiomyocyte, triggers transcriptional reprogramming (dedifferentiation), development processes activation involving cell cycle; multi-nucleation and a secretome that activate a developmental vascular cell remodeling program culminating in heart failure. We aim to unravel Wnt-dependent mechanisms instructing cardiovascular tissue formation, heart homeostasis and remodeling in a cell- and context-specific manner. Moreover, Transcription factor 7-like 2 (TCF7L2) is a major determinant of downstream Wnt target gene expression and is tightly controlled by tissue specific inhibitors. Thus, the Wnt nuclear pathway is a promising pharmacological target candidate for modulating the initiation of maladaptive heart remodeling and heart failure progression. However, pharmacological targeting of the Wnt pathway is challenging, because of its central role in many tissues. We have recently identified KLF15 as a cardiomyocyte specific regulator of Wnt/β-catenin signaling as well as defined its interaction as a tripartite complex associated with chromatin and the regulation of chromatin state. We are resolving the Wnt/β-catenin/KLF5 complex structure and stability as the basis for drug design.
Engineering synthetic transcription to control cell behavior
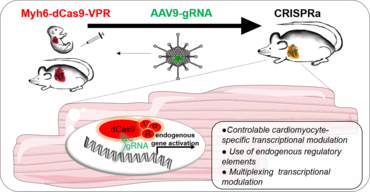
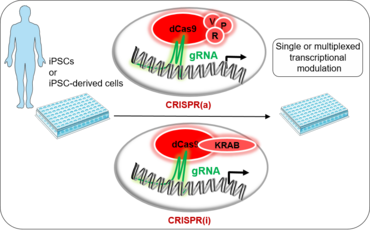
In order to find therapeutic concepts recovering the heart from damage, manipulation of multiple targets affecting gene regulatory networks (GRN) is required. In order to design such a therapy, fundamental cardiomyocyte biology and its cellular and molecular networks embedded in its in vivo natural environment need to be addressed. More importantly, this need to be elucidated in a disease-context-specific manner. CRISPR/Cas9 technology has been adapted to lose its catalytic activity completely and fused to transcriptional activators (CRISPRa) or repressors (CRISPRi) to modulate gene expression. CRISPRa / i has overcome limitations of previous transcriptional modulation methods. Importantly, it can be designed to create unprecedented levels of control offering a multiplexing possibility at the same time. Multiplexing allows modulating multiple genes within the same pathway thus potentiating a synergetic effect of tightly regulated endogenous GRNs. More importantly, the catalytic inactive Cas9 has limited unwanted effect leaving the genome unaffected, highly desired for therapeutic applications. All these elements will be exploited in our studies for deciphering complex endogenous mechanisms of regulation in cardiomyocyte disease biology.
Our methodology is based on biochemical, epigenetic, transcriptional and functional investigation of novel identified factors using classical mouse knockout strategies combined with mouse embryo electroporation (EP) culture, tissue explants and human induced pluripotent stem cells (IPSCs) and finally tissue engineered (in coll. With Prof. Zimmermann´s team). The used of human samples (heart biopsies and serum) are included to validated human relevant mechanisms. Our group has expanded the CRISPR-toolbox in mouse and in IPSCs. CRISPR/Cas9-based technology is used for gene editing (knockout and knockin) as well as for transcriptional modulation in vitro in in vivo.
Prizes
- 2020 - DZHK January 2020 Paper of the Month [here]
- 2019 - Teaching Award at the University Medical Center Goettingen (UMG)
- 2019 - Basic Science Poster Prize, German Cardiac Society (DGK) Basic Science Meeting awarded to Eric Schoger
Selected Publications
*denotes equal contribution
- Orr Shomroni, Maren Sitte, Julia Schmidt, Sabnam Parbin, Fabian Ludewig, Gökhan Yigit, Laura Cecilia Zelarayan, Katrin Streckfuss-Bömeke, Bernd Wollnik and Gabriela Salinas. A novel single‑cell RNA‑sequencing approach and its applicability connecting genotype to phenotype in ageing disease. Scientific Reports 12, 4091 (2022). PubMed PMID: 35260714.
- Yanpu Chen, Felipe F. Lüttmann, Eric Schoger, Hans R. Schöler, Laura C. Zelarayán, Kee-Pyo Kim, Jody J. Haigh, Johnny Kim,* and Thomas Braun. Reversible reprogramming of cardiomyocytes to a fetal state drives adult heart regeneration in mice. Science 373(6562):1537-1540 (2021) PubMed PMID: 34554778.
- Franziska S. Rathjens, Lavanya M. Iyer, Anke Renger, Fahima Syeda, Claudia Noack, Andreas Jungmann, Matthias Dewenter, Karl Toischer, Ali El-Armouche, Oliver J. Müller, Larissa Fabritz, Wolfram-Hubertus Zimmermann, Laura C. Zelarayán* and Maria-Patapia Zafeiriou*. Preclinical evidence for the therapeutic value of TBX5 normalization in arrhythmia control. Cardiovascular Research cvaa239 (2020). (*corresponding authors) PubMed PMID: 32777030.
- Schoger E, Carroll KJ, Iyer LM, McAnally JR, Tan W, Liu N, Noack C, Shomroni O, Salinas G, Groß J, Herzog N, Doroudgar S, Bassel-Duby R, Zimmermann WH, Zelarayán LC. CRISPR-mediated activation of endogenous gene expression in the postnatal heart. Circ Res 3;126(1):6-24 (2020) PubMed PMID: 31730408.
- Claudia Noack*, Lavanya M. Iyer*, Norman Y. Liaw, Eric Schoger, Sara Khadjeh, Eva Wagner, Monique Woelfer, Maria-Patapia Zafiriou, Hendrik Milting, Samuel Sossalla, Katrin Streckfuss-Boemeke, Gerd Hasenfuss, Wolfram-Hubertus Zimmermann, and Laura C. Zelarayán. KLF15-Wnt-dependent cardiac reprograming reveals a novel vascular player, SHISA3 in the mammalian heart. Journal of the American College of Cardiology 74(14):1804-1819 (*contributed equally) (2019) PubMed PMID: 31582141.
- Lavanya M. Iyer, Sankari Nagarajan, Monique Woelfer, Eric Schoger, Sara Khadjeh, Maria Patapia Zafiriou, Vijayalakshmi Kari, Jonas Herting, Sze Ting Pang, Tobias Weber, Franziska S. Rathjens, Thomas H. Fischer, Karl Toischer, Gerd Hasenfuss, Claudia Noack, Steven A. Johnsen and Laura C. Zelarayán. A context-specific cardiac Beta-catenin and GATA4 interaction influences TCF7L2 occupancy and remodels chromatin driving disease progression in the adult heart. Nucleic Acids Research 46(6):2850-2867 (2018) PubMed PMID: 29394407.
- Noack C, Zafiriou MP, Schaeffer HJ, Renger A, Pavlova E, Dietz R, Zimmermann WH, Bergmann MW, Zelarayán LC. Krueppel-like factor 15 regulates Wnt/beta-catenin transcription and controls cardiac progenitor cell fate in the postnatal heart. EMBO Mol Med 4: 992-1007 (2012) PubMed PMID: 22767436.
Members Zelarayan Group
Alumni
- Dr. Federico Bleckwedel
- Dr. Elena Chebbok
- Dr. Lavanya Iyer
- Gabriela Maria Lobo Vallejo
- Dr. Claudia Noack
- Dr. med. Laura Priesmeier
- Dr. Franziska Rathjens
- Dr. Anke Renger
- Dr. Cheila Rocha
- Dr. Eric Schoger
- Dr. Monique Wölfer